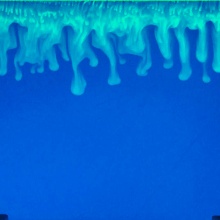
New SFB 1313 publication, published in Water Resources Research. The paper has been prepared within SFB 1313's new vision topic on precipitation and dissolution of salt/lime in porous media.
"On the role of density-driven dissolution of CO2 in phreatic karst systems"
Authors
- Holger Class (University of Stuttgart, SFB 1313 research project C04)
- Pascal Bürkle (University of Stuttgart)
- Tim Sauerborn
- Oliver Trötschler (University of Stuttgart)
- Bettina Strauch (GFZ Potsdam)
- Martin Zimmer (GFZ Potsdam)
Abstract
Density-driven dissolution of carbon dioxide in water is a well-known and much described mechanism in geological sequestration of this greenhouse gas. It is remarkable that such enhanced dissolution does not receive attention in karst hydrology and speleology. Models and hypotheses on karst development are complex and consider many different processes. We focus here on the influence of CO2 partial gas pressures at the interface between atmosphere and karst water on the dynamics of dissolved CO2 concentrations below the water table. Seasonal variations of microbial soil activity and root respiration or barometric-pressure changes cause fluctuations in CO2 partial pressures. Dependent on the existence and strength of a karst-water background flow, fingering regimes might be triggered causing enhanced dissolution of CO2. This allows replenishment of CO2, and, thus, dissolutional power even deep in the water body without the need for percolating water to transport dissolved CO2. We present and discuss simplified and generic experimental and computational scenarios to strengthen our claim, and we try to give answers to: how much? and under which circumstances? The applied numerical model solves the Navier-Stokes equation with water density dependent on CO2 concentration and temperature. We show that calculated CO2 mass fluxes into the water bodies are dependent on the ratio of Péclet to Rayleigh numbers (Pe/Ra) and show a local minimum around Pe/Ra = 1, i.e. when natural and forced convection are about equal. Concluding, we claim that there is sufficient reason to consider density-driven dissolution as a process of relevance in karstification if circumstances are given.

Holger Class
apl. Prof. Dr. Ing.Project Leader, Research Project C04, Central Project Z